Dr. Lars Erik Rutqvist of Swedish Match AB: Phase III Clinical Trials on Swedish Snus began in 2008!

Swedish Match AB to pursue FDA ‘Reduced Harm Product’ Designation
Andrew Romeo recently wrote a column ‘Philip Morris says Snus will not Help Smokers Quit.’ I’m not going to repeat it here but encourage you to read it if you have not done so already. He eviscerates the nature of the PMUSA-sponsored study, the quality of the study, and in doing so demonstrates how the Study was pre-destined to produce the results Philip Morris USA desired: that snus was a complimentary product; not a replacement for cigarettes.
In short, the PMUSA Study was designed to support their marketing strategy; not the facts. At least their motivation is transparent.
Others just choose to ignore the facts if they are inconvenient. Facts never stand in the way of zealots; especially those anti-all-tobacco zealots.
Despite over 100 published scientific studies between 1980 and 2009 on 450,000 long-term Swedish Snus users and cancer, cardiovascular disease, metabolic effects and more…..
Despite the living laboratories of Sweden and Norway which have clearly shown the correlation between Swedish Snus and Smoking Cessation…..
Despite the data from just one of these studies; the ITS//FSI study by Ramström & Foulds 2006 which demonstrated the following data….. It all just wasn’t good enough.
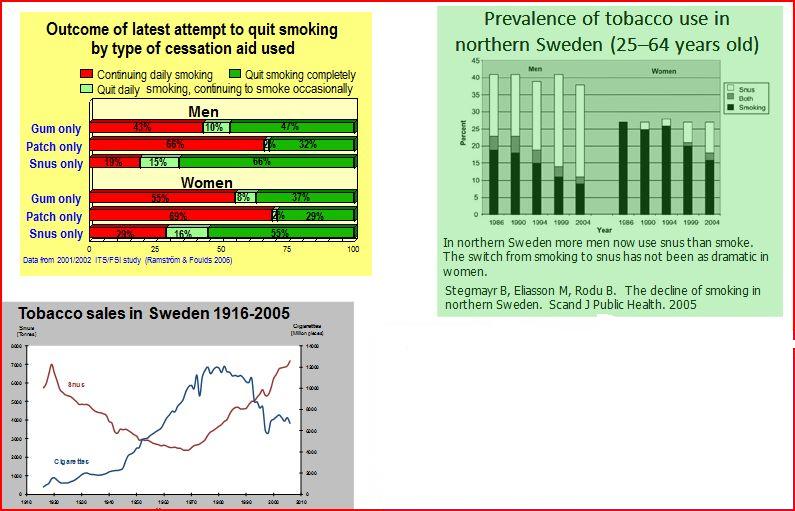
Despite the more-than-conservative assertion by Dr. Lars Erik Rutqvist that “as a result of convincing evidence from analytical epidemiological studies there is today a consensus with the scientific community that Smokeless Tobacco use is associated with substantially less health hazards than smoking (e.g. cancer, chronic lung disease, cardiovascular disease, and metabolic disease), although estimates of the reduction in risk varies considerably: from 50% to 99.9%.”……..
The facts JUST weren’t good enough or convenient enough for some.
– “I cannot conclude that the use of any tobacco product is a safer alternative to smoking.”
– “As Surgeon General I cannot recommend use of a product that causes disease and death as a ‘lesser evil’ to smoking.”
– “….there is no scientific evidence that smokeless tobacco products are both safe and effective aids to quitting smoking.”
– “Smokers…should Not trade on carcinogenic product for another, but instead could use FDA-approved methods such as nicotine gum, nicotine patches, or counseling.”
The above four comments were made by then-U.S. Surgeon General Richard Carmona in 2003. Obviously a non-smoker as well as an ideologue.
Others such as SCENIHR, the WHO Tobacco Regularity Committee, and Hom et al in journals of the five Nordic Medical Associations, hedged their bets in various published articles and reports during 2008:
– “no randomized trial has been conducted”
– “Some observation studies….results are inconsistent”
– “On the available evidence…not possible to draw conclusions as to the relative effectiveness ….in comparison with established therapies.”
– “The evidence that smokeless tobacco products are effective for smoking cessation does not meet the standards required…”
– “There are evidence-based methods for smoking cessation…There is no scientific documentation on the effect of snus as a smoking cessation aid.”
None said it clearer than Matt Myers of the supposed Campaign for Tobacco-Free Kids, the folks that helped Philip Morris write the embarrassment of the Kennedy/Waxman legislation; now law, when Mr. Barry said in 2004:
“If these companies think their products are so safe, so safe in fact that they even claim they can be used to help people quit smoking, then there is nothing, and I repeat, absolutely nothing, stopping them for going to the Parkland Building in Bethesda where the FDA has its headquarters and submitting a new drug application for their products as a smoking cessation device. UST and Swedish Match say that they have the scientific evidence to back this claim. It is long past the time for these companies to put their science where their mouths are (and the obigitory and totally off-topic ending,) and stop targeting our kids.”
Actually Matt, there was a lot stopping them back then but the facts never got in your way. Ironically, those barriers are lifted somewhat by the same legislation CTFK and Philip Morris helped write and lobby into The Family Smoking Prevention and Tobacco Control Law…Kennedy/Waxman.
In giving FDA regulatory authority over tobacco products, a new category was created: “Modified Risk Products”. MRP products break down in two classifications; “Reduced Exposure Products” and the more desirable “Reduced Harm Products”.
Swedish Match AB, the largest manufacturer of Swedish Snus in the world decided to resolve these questions back in 2007; at least for Swedish Match snus products. How? The same way the drug companies do when they want FDA approval for a new drug; with very expensive Phase III Clinical Trials. I’m sure Matt Barry is more horrified than proud that SMAB sort of took his advice.
In this case, Two independent, randomized, placebo-controlled, double-blind clinical trials conducted according to GCP testing Snus plus Counselling versus Counselling alone.
The aim of these clinical trials is multi-fold. First, to produce conclusive scientific evidence of the ability of snus to increase quit rates among cigarette smokers; a smoker can quit cigarettes by switching to smokeless tobacco.
Then in conjunction with other evidence, be able to show FDA that Swedish Match/GothiaTek Standards-produced Snus qualifies to be classified as a Reduced Harm Product. This would include that Swedish Match Snus:
- Is substantially less hazardous than cigarette smoking.
- Is not a gateway to smoking
- Availability/FDA endorsement does NOT
- decrease population smoking cessation rates
- result in “unnecessary” tobacco use (smokers who would have quit anyway, or snus users who otherwise would not have used any tobacco products)
- obscure society’s anti-tobacco message
In the very complex conceptual world of ‘Tobacco Harm Reduction’ three words: “Proof of Principle”.
 In an exclusive presentation and interview with Lars Erik Rutqvist, M.D., Ph.D, and Senior Vice President for Scientific Affairs for Swedish Match AB back in June before Kennedy/Waxman had become law (which I doubt I will receive a well deserved Pulitzer Prize for…tobacco politics), Dr. Rutqvist revealed that beginning in 2008, two independent Phase III Clinical Trials which are described in the US as “Randomized, Placebo-Controlled, Double-Blind Clinical Trials of a Smokefree Tobacco Product (Snus) to increase the Quit Rate Among Cigarette Smokers Who Wish to Stop Smoking”, began.
In an exclusive presentation and interview with Lars Erik Rutqvist, M.D., Ph.D, and Senior Vice President for Scientific Affairs for Swedish Match AB back in June before Kennedy/Waxman had become law (which I doubt I will receive a well deserved Pulitzer Prize for…tobacco politics), Dr. Rutqvist revealed that beginning in 2008, two independent Phase III Clinical Trials which are described in the US as “Randomized, Placebo-Controlled, Double-Blind Clinical Trials of a Smokefree Tobacco Product (Snus) to increase the Quit Rate Among Cigarette Smokers Who Wish to Stop Smoking”, began.
They both involve using two groups of participants at each each study center: one group is using Swedish Match Snus in either 1.0 gram or 0.5 gram portion pouches; the other using the same tasting and weight of pouches but filled with a Swedish Match Onico product (tobacco/nicotine free).
Serbia and the United States were chosen as the countries for the trials; the United States for obvious reasons and Serbia since it has a very high smoking rate which has become almost cultural. Dallas, TX was just added as the 6th American trials location. Fortunately for all of us, details of the Swedish Match Trials are finally publicly available on the US National Institues of Health Website as SM-07-01 for the Serbian trials and SM-08-01 for the American trials.
In my interview with Dr. Rutqvist, which I again stress for reasons you will soon see, occurred prior to President Obama signing Kennedy/Waxman into law, I asked Dr. Rutqvist about the provision in The Family Smoking Prevention and Tobacco Control (not!) legislation which prohibits manufacturers from even talking about Reduced Harm in conjunction with their products.
“Since there will be a regulatory route in the US legislation for Reduced Harm Products, we interpret that once the Kennedy/Waxman legislation is law, to imply that a manufacturer cannot make reduced harm claims in their communication unless their product has been approved by the FDA. FDA can take up to 2 years to present their interpretation of the law text as to what is required to show reduced harm status.
A manufacturer can obviously not submit an application for a reduced harm product until the exact criteria have been established. Preparation of an application and the review by FDA of that application obviously also take some time.
This means that we cannot expect any product to be approved until realistically 3-4 years from now.“
Thank you Philip Morris USA. They don’t like the Reduced Harm Product classification as they are all about Marlboro  cigarettes.
cigarettes.
How is the new Marlboro Blend No 54 doing since you made sure banning of menthol was not within FDA’s new powers? How many millions will ultimately die unnecessarily because of the 3 to 4 years Dr. Rutqvist estimates it will take for the first Reduced Harm Product applications to be approved? Thanks to you, is Dr. Rutqvist even going to be allow to answer his emails or phone for the next few years?
I was also curious if Swedish Match did ultimately obtain the Reduced Harm Product designation from FDA, would these Phase III Clinical Trials only apply to Swedish Match/GothiaTek Standard products, Swedish Snus in general, or smokeless tobacco across the board?
Dr. Rutqvist admitted “We don’t know how the FDA will view there things. That said, I would be very surprised if they were to issue a ‘generic’ approval of ‘snus’ given the fact that there is no universally accepted definition of what constitutes ‘snus’.
We expect the FDA to approve only a specific product, with a defined content, properties, usage pattern, and risk profile. We have no idea how ‘wide’ a potential approval may turn out to be, but we expect it to be quite narrow. The snus products used in our trials were manufactured at our Gothenburg facility and complies with the GothiaTek standard.”
So your studies and application will not be a boiler-plate for other Swedish/Scandinavian snus manufacturers and especially, so-called American Snus, to qualify for a Reduced Harm classification by FDA?
“The trials will probably be of no use to manufacturers of products that are ‘substantially’ dissimilar to Swedish Match products. We don’t know how the FDA will view products that are ‘substantially’ similar to ours. In the pharmaceutical world, ‘generic’ status requires a product to be identical to the original product. We don’t know how this will play out when it comes to tobacco given the chemical complexity of any product based on plant material. Even in our products there may be slight variations over time as a result of differences in the raw tobacco used.”
Which of course begged the question, since Swedish Match manufactures it, will Triumph Snus automatically be included in your Reduced Harm application to FDA or will Lorillard have to submit their own petition attempting to use your clinical trials as evidence?
Dr. Rutqvist smiled and replied “At this moment, I simply don’t know.”
I quickly followed up by asking the impact the clinical trials could have on or affect classification of Swedish Match’s American Smokeless Tobacco/non-snus brands like Red Man?
He smiled again and said “We don’t know”.
 Now it was my turn to smile. Welcome to the American legislative process. Our Congressmen and Senators vote on bills they admit not having the time to read; written by lobbyists for Congress as Congress doesn’t have the time to write the bills themselves, and are signed into law by the President who also hasn’t read the document cover to cover and in this case, signed more to honor poor Ted Kennedy who was hospitalized again at the time then to seriously address cigarette smoking in this country.
Now it was my turn to smile. Welcome to the American legislative process. Our Congressmen and Senators vote on bills they admit not having the time to read; written by lobbyists for Congress as Congress doesn’t have the time to write the bills themselves, and are signed into law by the President who also hasn’t read the document cover to cover and in this case, signed more to honor poor Ted Kennedy who was hospitalized again at the time then to seriously address cigarette smoking in this country.
All the politicos in Washington smile for the cameras, use the words “for the children” a lot, and continue on to the next photo op. Ultimately it is not the elected officials but the life-long bureaucrats who fill in the details of these bills. Often it takes many years.
In the case of The Kennedy/Waxman Law, the SnusCENTRAL Intelligence Agency is already aware of more-than-one lawsuit in the wings challenging the law on First Amendment violations. Who knows how many more will be generated once FDA starts filling in the blanks. More time. More money…our money…down the drain.
EU politics can be no less dispiriting, but this is America: now home of trillion dollar deficits, vacuous legislation, a 9.5% (as of this writing and climbing) Unemployment Rate, and in Texas, Toll Roads as far the the eye can see in just about any direction.
Happy 2009. Wait until you see 2010. At least some of us have our delicious Swedish Snus to take the sting out….for now.
LARRY WATERS
Swedish Snus Ambassador to the United States
Reporting From Sweden and the USA for SnusCENTRAL.org
About author
You might also like
Snus News: Snus Prices on the Rise
BREAKING SNUS NEWS……………….. The SnusCENTRAL Intelligence Agency office in Stockholm just wired critical information concerning yesterday’s rumors of an increase in the wholesale price of Swedish Snus. The source of
EXCLUSIVE: Triumph Snus Manufacturer reveals Triumph Snus is LOW NICOTINE: Lorillard Senior Official Refuses to Comment!
EXCLUSIVE Breaking SNUS News! It appears that the second of the Top Three US Tobacco Companies has been caught intentionally cutting nicotine levels in their snus products to keep their
FDA Approves Eight Swedish Match Smokeless Tobacco Products
Today, FDA announced it was authorizing the sale and marketing of eight Swedish Match smokeless tobacco products in the US. These first eight are not in the snus category but





