Unregulated Tobacco Products: A Victory for Tobacco Users or an FDA Trap?
 March 23rd 2011, FDA announced to a stunned tobacco and anti-tobacco community that they had decided Star Scientific’s new BDL line of nicotine-containing smokeless tobacco lozenges (artificial snus as I call them) were really not considered nicotine-containing smokeless tobacco under the Family Smoking Prevention and Tobacco Control Act (Tobacco Control Act for short).
March 23rd 2011, FDA announced to a stunned tobacco and anti-tobacco community that they had decided Star Scientific’s new BDL line of nicotine-containing smokeless tobacco lozenges (artificial snus as I call them) were really not considered nicotine-containing smokeless tobacco under the Family Smoking Prevention and Tobacco Control Act (Tobacco Control Act for short).
The Star BDL dissolvable products contain reduced levels of carcinogens known as tobacco-specific nitrosamines or TSNA. Star Scientific said it was notified by FDA Tobacco Czar Lawrence Deyton that “cigarettes, cigarette tobacco, smokeless tobacco and roll-your-own tobacco” are the only products subject to regulation under the 2009 Tobacco Control Act.
Jumping ahead to April 25th, FDA Tobacco Czar Lawrence Deyton announced the decision by FDA not to appeal Judge Richard Leon’s ruling in NJOY v FDA to the US Supreme Court. This means that e-cigarettes, e-liquid, nicotine gums, lozenges, patches, skin creams and nasal sprays are now unregulated tobacco products according to federal law (as long as no therapeutic claim is made by the manufacturer/importer). These nicotine products now join cigars and pipe tobacco as unregulated tobacco products under the infamous Family Smoking Prevention Tobacco Control Act.
During my “unscheduled interview” with Director Deyton at NATO Show 2011 two weeks ago, I challenged him to explain how it was in the interests of public health for Swedish Snus to be mis-characterized with factually incorrect warning labels as a dangerous high TSNA cancer causing product. His response concerning Expedited Review stunned me. You can read the complete interview in my article on NATO Show 2011 for more details.
Reactions to all the above ranged from outrage and horror from the Anti All Tobacco Extremists to silence from Big Pharmaceutical to optimism from the Pro Reduced Harm Tobacco Product community.
Bill Godshall, executive director of Smokefree Pennsylvania, welcomed this sea change in the federal legal definition and regulation of tobacco/nicotine products, stating: “Smokers will have greater access to many different less expensive smokefree alternatives, and manufacturers can now truthfully market these new smokefree tobacco products to smokers as far less hazardous alternatives to cigarettes.”
The change is also likely to decimate drug industry sales of nicotine gums, lozenges and patches. “Not many smokers will pay $.75 for a nicotine lozenge if they can buy a virtually identical tobacco lozenge for $.20,” added Godshall.
As for myself, I was also hopeful. I even suggested that Swedish Match AB should leap upon recent events as an opportunity to break low-TSNA Swedish Snus out of the generic smokeless tobacco category by using Director Deyton’s Expedited Review process to either have Swedish Snus reclassified as an Unregulated Tobacco Product or at least as a less regulated Modified Risk Tobacco Product.
Despite my shared optimism with Bill Godshall, I was also very uneasy. All of these events were completely unexpected and frankly seemed too good to be true. I’ve been waiting for the other shoe to drop.
On April 22nd, the day prior to Director Deyton declaring his decision that Star’s BDL products don’t fall under the Tobacco Control Act, deep-cover SnusCIA Agents in Washington obtained a copy of an FDA memo which was sent to the members of the Tobacco Products Scientific Advisory Committee (TPSAC). Below is that memo from Director Deyton.
The Leaked Memo from TPSAC and FDA Tobacco Czar Deyton
TPSAC
To
cc
Subject Message from Lawrence R. Deyton, MSPH, MD
TPSAC members:
Thanks again to all of you for your hard work on the Menthol Report. Now we move on to the next very important area of work – dissolvable tobacco products. Clearly we will be back in the spotlight addressing the many varied challenges that dissolvable tobacco products will bring to the table.
As we transition from menthol and onto dissolvables and other TPSAC work, I want to make sure that you know that David Ashley and his staff in the Office of Science will be leading and coordinating the TPSAC work on dissolvables and other topics in the future. TPSAC is our science advisory committee and thus, organizationally, is supported by the Office of Science.
Corinne Husten took the first lap for the Center with menthol because she has relevant experience and represented CTP’s overall public health perspectives addressing the menthol issues. And, frankly, she was the only one of our early senior staff who I could depend on to handle the complexities of simultaneously establishing TPSAC and launching the work on menthol.
Now that David Ashley is on board and has begun to build a hearty staff, the stewardship of TPSAC needs to fully transition to him and the Office of Science.
Corinne is Senior Medical Advisor in the Office of the Director and her role will continue to be the public health voice for CTP in all kinds of endeavors, including TPSAC. However, she will not be coordinating or leading the TPSAC work for CTP.
David has designated Dr. Sarah Evans as the lead for the Office of Science TPSAC work on dissolvables. As with menthol, there are other members of the Office of Science staff who will continue to support the committee. This shift in coordination of TPSAC within CTP has been planned for about 9 months. It is happening now because it is a natural transition point in the work of TPSAC and because the Office of Science is now properly staffed for this work.
We very much look forward to the continuing work of the Advisory Committee – there is much important work ahead. Please feel free to contact Caryn Cohen, MS, Designated Federal Official for TPSAC, if you have any questions.
Lawrence R. Deyton, MSPH, MD
Sent for Dr. Deyton by Caryn Cohen, M.S.
[redacted] or tpsac@fda.hhs.gov
Center for Tobacco Products/FDA
This memo is not a smoking gun per se: you have to read between the lines a little. Deyton starts by thanking everyone for their hard work on the menthol report. TPSAC’s overall recommendation on menthol was “Removal of menthol cigarettes from the marketplace would benefit public health in the United States.”
In responding to media questions, Deyton remarked that “receipt of the Report does not have a direct immediate effect on the availability of menthol products in the marketplace.” Deyton further noted: “Although there is no required deadline or timeline for FDA to act on the issue of menthol in cigarettes, we recognize the strong interest in this issue among all stakeholders and will continue to communicate the steps the FDA is taking as it determines what future regulatory actions, if any, are warranted. FDA intends to provide its first progress report on the review of the science in approximately 90 days.“
Armed with new insights into Director Deyton from NATO Show 2011, his comments on the menthol report are not as benign as they first may sound. Operationally, I’ve concluded Deyton is a classic bureaucrat. He carefully builds a case to his liking under the framework in which Congress gave him to operate. He will not rush ahead. He will make sure that the entire process is carried out exactly by the book. This way, when the time comes to make a final ruling, no one can challenge it as biased, violating the process, or the law.
At the same time, TPSAC in particular and the FDA CTP staff has been loaded with anti-all-tobacco extremists. In the case of TPSAC, a blind-sided Altria (who helped write the Tobacco Control Act) petitioned for certain members of TPSAC to be replaced for their documented anti-tobacco positions. Director Deyton didn’t see a problem with these TPSAC members and refused.
Now the process is beginning for dissolvable tobacco products which would include, one would think, not only Camel Orbs but Star Scientific’s newly emancipated BDL series of lozenges. Brian J. Malkin of Frommer Lawrence & Haug LLP concluded in an article for the FLH Tobacco Blog that “According to the Tobacco Control Act, TPSAC’s next report, due a year from now, will be on dissolvable tobacco products. As the Menthol Report is now the model, one might suspect a similar approach will be followed, perhaps with a similar set of conclusions and recommendations.“
Regarding the new suddenly Unregulated Tobacco Products, the FDA has already indicated that it plans to propose regulations for these smokefree nicotine alternatives as tobacco products. I suspect that these proposed regulations, no doubt to be made by TPSAC, will be far from friendly to any nicotine containing product not made by Big Pharmaceutical. Dr. Deyton also stated that formerly sacrosanct cigars may (meaning will) be reviewed by TPSAC some time in the future as well.
While the events of the last month appear to be battle field victories in the War Against Tobacco, they may simply be strategic retreats by the anti-all-tobacco extremists to ensure their eventual victory of the entire Tobacco War. The tobacco and nicotine products industry should take strong advantage of this situation while they still can. We have short window of opportunity deserves a full-court press now. Within a year or so, that window and maybe even the Tobacco War will be all but decided. It’s time for the industry to stop cowering in fear of the next FDA Advisory. It is time to charge forward and attack.
Reporting from the Front Lines,
LARRY WATERS
Swedish Snus Ambassador to the United States
Reporting for SnusCENTRAL.org

About author
You might also like
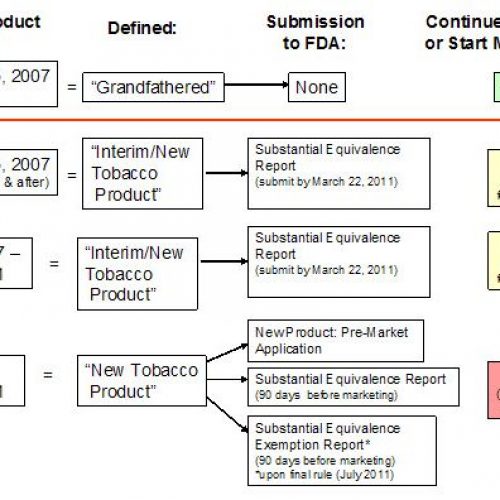
FDA Tobacco Substantial Equivalence – Could your favorite brand be banned?
In a conference call with the tobacco industry (and me) earlier today, FDA announced they would begin to enforce the Substantial Equivalence provisions of the Family Smoking Prevention and Tobacco
S1147: The PACT Act. A Date Which will Live in Infamy
As the sun rose steadily across the United States yesterday, it seemed a day like any other day. Children dropped off at school, parent(s) off to work or to the
Snus Psychology at Work in The Lab at Swedish Match
Psychology has always played a major roll in product development and marketing. Words like bigger, stronger, faster, better, and safer are only a few of hundreds of examples. Read the